Genomic Insights into Mangrove Rhizosphere Fungi: Understanding Microbial Adaptations in Coastal Ecosystems
Mangrove forests are among the most biologically diverse and ecologically productive ecosystems, hosting a rich array of plant, animal, and microbial life. Fungi, particularly those residing in mangrove rhizospheres, play a crucial role in organic matter decomposition, nutrient cycling, and symbiotic interactions with plants. However, their genomic characteristics remain largely unexplored. In our recent study published in Frontiers in Fungal Biology, we present draft genomes and comparative analysis of seven fungal species isolated from mangrove rhizospheres, offering key insights into fungal adaptation and metabolic potential in these challenging coastal environments.
Key Findings
-
Genome Sequencing and Assembly:
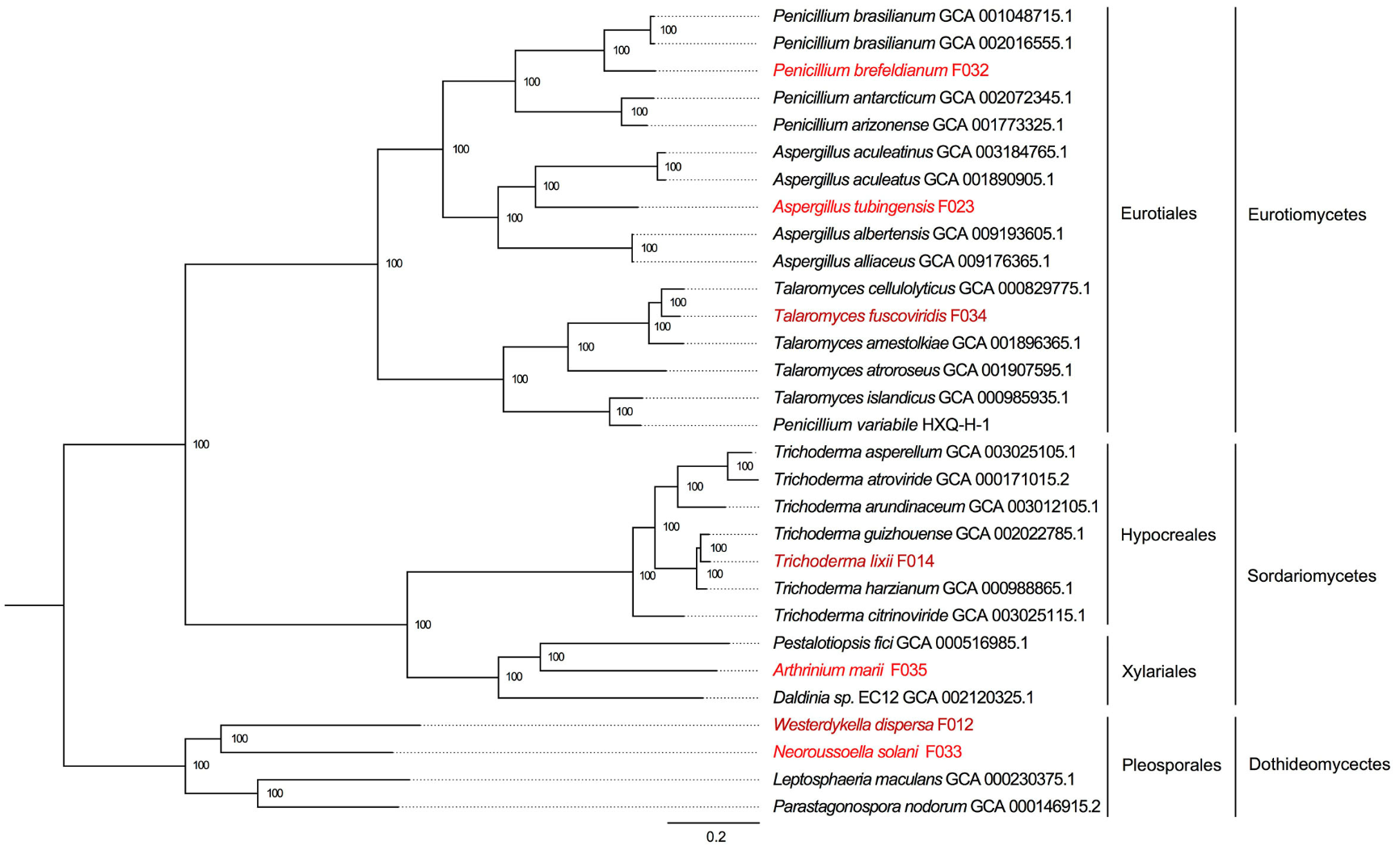
Seven Ascomycota fungi were isolated from the rhizospheres of Kandelia obovata and Acanthus ilicifolius, two dominant mangrove species. Using high-throughput sequencing, we assembled fungal genomes ranging from 29 to 48 Mb, achieving high gene completeness (>94%). - Carbohydrate Metabolism and Environmental Adaptations:
Comparative genomics revealed key differences in carbohydrate-active enzymes (CAZymes) between mangrove-associated fungi and non-mangrove fungi. Notably:- The CBM32 enzyme family was exclusively present in two mangrove fungi, potentially contributing to unique polysaccharide degradation mechanisms.
- Significant variation in GH6 (cellobiohydrolase) and PL4 (rhamnogalacturonan endolyase) gene families was observed between fungal species from the two mangrove hosts, suggesting host-specific metabolic adaptations.
- Secondary Metabolite Biosynthesis and Bioactive Potential:
We annotated gene clusters involved in secondary metabolite (SM) biosynthesis, finding that mangrove fungi harbor significantly higher numbers of Type I Polyketide Synthase (t1pks) genes compared to non-mangrove fungi. These findings underscore the potential of mangrove fungi as sources of bioactive compounds, which may contribute to microbial competition and environmental resilience.
Reflections
This study represents an important step in XinLab’s research on the genomic basis of mangrove symbiotic microorganisms, supported by a collaborative research grant with Professor Simon Lee’s team at the University of Macau. As part of our broader efforts in environmental genomics, we focused on characterizing microbial genomes in mangrove habitats—leveraging our expertise in bioinformatics and sequencing technologies. By isolating microbial strains, sequencing their genomes, and analyzing biosynthetic pathways, we uncovered valuable insights into microbial adaptation strategies, metabolic diversity, and bioactive compound production.
This project holds personal significance, as it highlights how genomics can deepen our understanding of mangrove-associated biodiversity and contribute to the conservation and sustainable use of mangrove ecosystems. As the study progressed, I reflected on how our work bridges modern sequencing techniques with ecological insights—revealing the interconnectedness of microbial life and plant symbiosis in coastal environments. While this study lays the foundation for further exploration, we are already pursuing additional genomic research on mangrove microbiomes across different regions, aiming to expand knowledge on their ecological roles and applications.
The full text of this study can be accessed online at Frontiers in Fungal Biology.